In Which Soluion Does Fe Oh 2 Dissolve Best
A stability field diagram shows the Eh and pH values at which each of these predominates. The answer is no.

Inorganic Chemistry Is Fe Oh 2 Soluble In Alkalis Chemistry Stack Exchange
FeOOH is soluble in acids giving FeH2O63.
. Form a green precipitate with green colour solution. The net reaction of CaF 2 with strong acid is thus. The solubility in water of a compound depends inversely on the lattice energy of the compund.
Important ionic species present include Fe FeOH FeOH2 Fe and FeOH. Write a balanced equation for the solubility equilibrium. The value of Ksp for FeOH2 80 x10-10.
Solubility refers to how much a substance will dissolve in a solvent. HOW CAN U TELL IF HNO3 KNO3 IS A BUFFER SOLUTION A buffer solution must contain a weak acid and its conjugate base OR a weak base and its conjugate acid. The addition of S2- ion to FeOH2s results in the formation of FeSs.
Iron tablets primarily consist of the hydrated form of iron II sulphate also known as ferrous sulphate which has a chemical formula of FeSO4. 2NaOH FeCl2 FeOH2 green ppt 2NaCl. Why does FeOH 3 dissolve in aqueous sulfuric acid.
Many metal ions form ferrocyanide precipitates so potassium ferrocyanide is not a good reagent for separating metal ions. Some FeOH2 precipitates. Form a green precipitate with green colour solution.
The potassium compound will react with hydrochloric acid. If the mixture is filtered the colourless filtrate youll get is sodium chloride NaCl solution. 1 What is the maximum concentration of ferric hydroxide that can dissolve.
This ion along with others may be precipitated with the chloride ion. Does ZrO OH2xH2O dissolve in HCl. A solution of the potassium compound will form a precipitate when added to an FeCl2 solution.
C hydroxide ions formed by the dissolution of the sparsely soluble iron salt are neutralized by acid. Click hereto get an answer to your question Which one of the following metallic hydroxides does not dissolve in sodium hydroxide solution1. A sample is tested for the presence of the Hg22 ion.
Thus it will dissolve readily in a dilute solution of a strong acid such as HCl and also in a solution of an alkali such as sodium hydroxideIt can be. Write the expression for the solubility product constant Ksp and calculate its value. B The K sp for the sparsely soluble iron salt is increased in magnitude by the addition of the aqueous sulfuric acid.
Form a white or pink precipitate. Although it is an ionic compound it does not dissociate into its ions in. CaF2 s 2H aq Ca2 aq 2HFaq Example 1871 shows how to calculate the solubility effect of adding a strong acid to a solution of a sparingly soluble salt.
FeOH2 is ferrous hydroxide or iron hydroxide. A sulfuric acid neutralizes excess hydronium ions in the sparse soluble iron salt mixture. Atmospheric oxygen is doing the oxidation I think of FeOH 2 to FeOH 3 which is even less soluble- this is why its purifying the MnOH 2 - which somehow dissolves and is left in solution with the chloride ions.
Answer 1 of 2. Sodium hydroxide also produces FeOH2and FeOH3from the corresponding oxidation states of iron in aqueous solution. Neither hydroxide precipitate dissolves in excess sodium hydroxide.
Now lattice energy is inversely proportional to the size of cations and size of anions. Ferrous hydroxide will be forced out of the solution as a precipitate because it isnt soluble in water. HNO3 is a strong base and KNO3 is the salt of a strong base KOH and a.
Form a brown precipitate with brown colour solution. FeOH3 aq may be present as part of the dissolved iron in natural water at alkaline pH and Fe OH2 aq may exist at pH 10 and above. Calculate the pH of a saturated solution of FeOH2 at 25C.
In concentrated aqueous alkali Fe2O3 gives FeOH63. Ferric Hydroxide Fe OH3 is an insoluble salt with a solubility of 10-39. It looks like it could be a complex combination of reactions which my best guess is something along the lines of.
That green precipitate is actually ferrous hydroxide FeOH2. The equation for the reaction is. Is FeOH2 Iron II hydroxide soluble or insoluble in water.
Stack Exchange Network Stack Exchange network consists of 179 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and build their careers. The potassium compound is water-soluble. Explain why the addition of S2- ion to CrOH3s does not result in the formation of Cr2S3s.
As the anions are same in all the 3 compunds. Sparingly soluble salts derived from weak acids tend to be more soluble in an acidic solution. But why ligand exchange reaction does not occur for FeOH2.
1 All iron sulphates dissolve in water to give the same aquo a complex ion containing one or more water molecules complex M H2Onz - in this case Fe H2O62. 1 The solubility of iron II hydroxide FeOH2 is 143x10-3 gram per liter at 25C. The hydrolysis was performed by simply refluxing the.
We attempted synthesis of ZrO2 powders starting with the hydrolysis of ZrOCl2 in aqueous solution. 2 Using Le Chataliers Principle explain why iron III solutions are more soluble in acidic solution.

Ap Solubility Equilibrium Free Response Questions

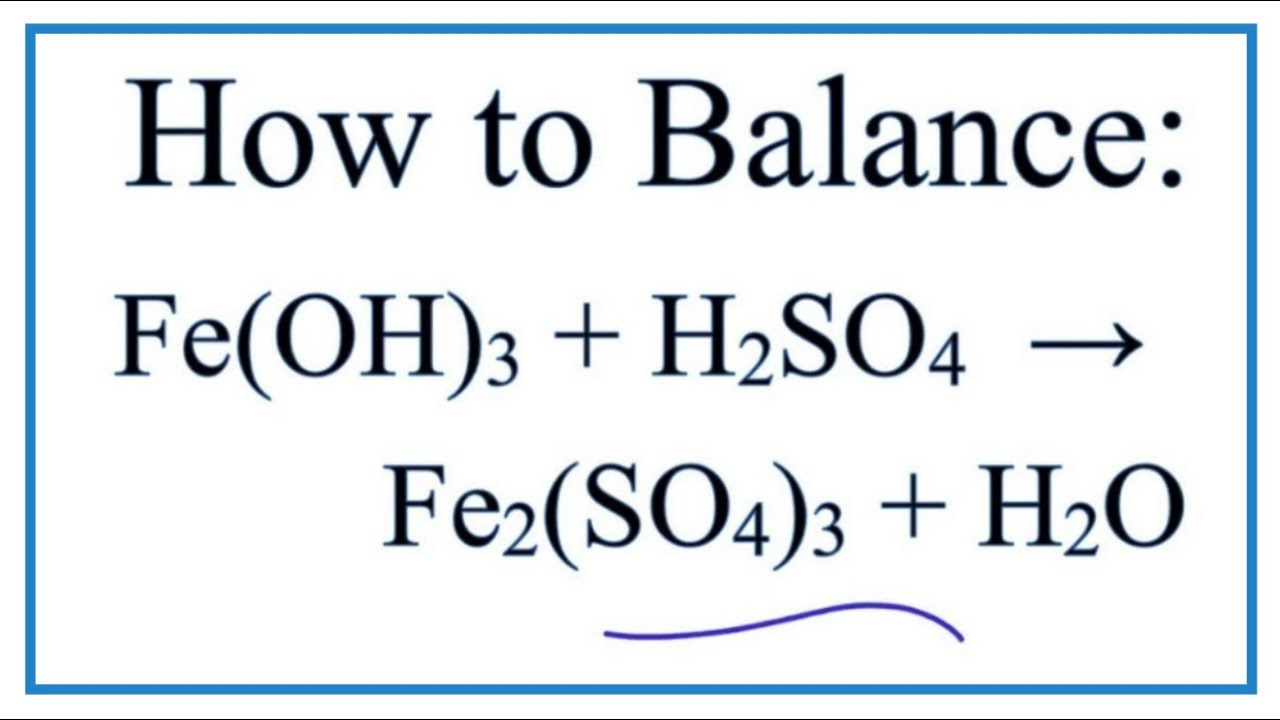
Is Fe Oh 3 Soluble Or Insoluble In Water Youtube
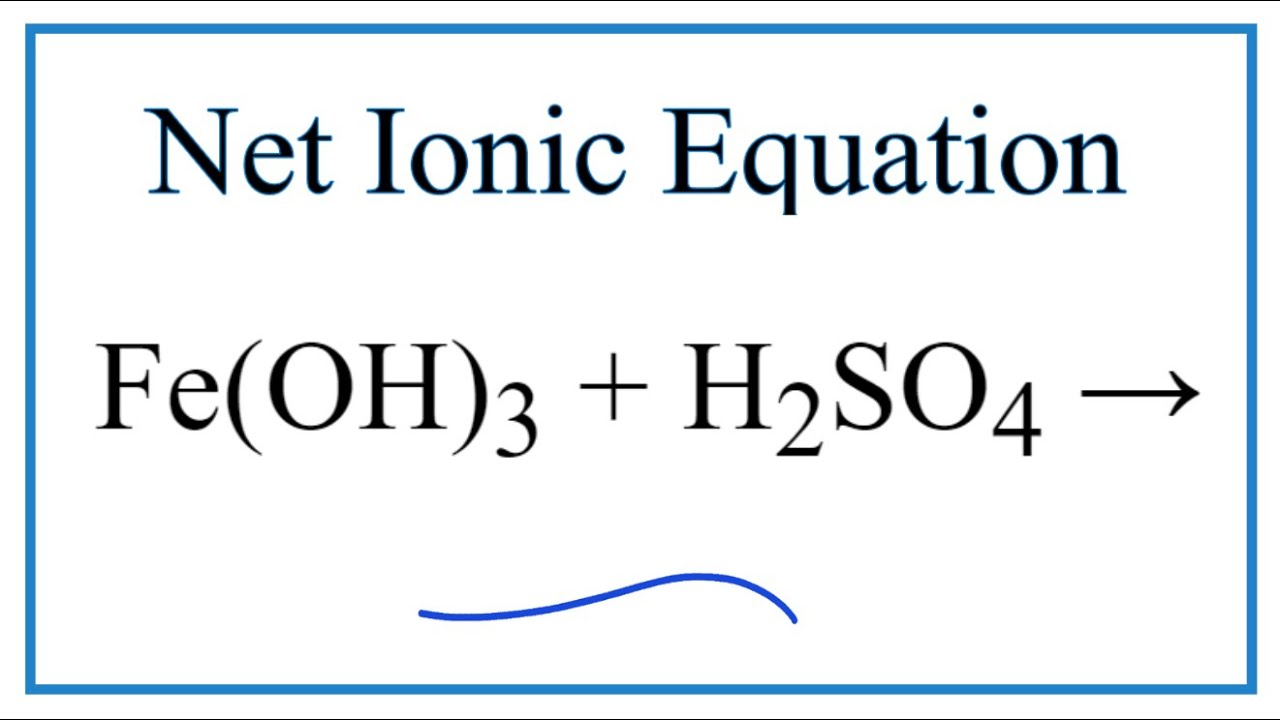
How To Write The Net Ionic Equation For Fe Oh 3 H2so4 Fe2 So4 3 H2o Youtube
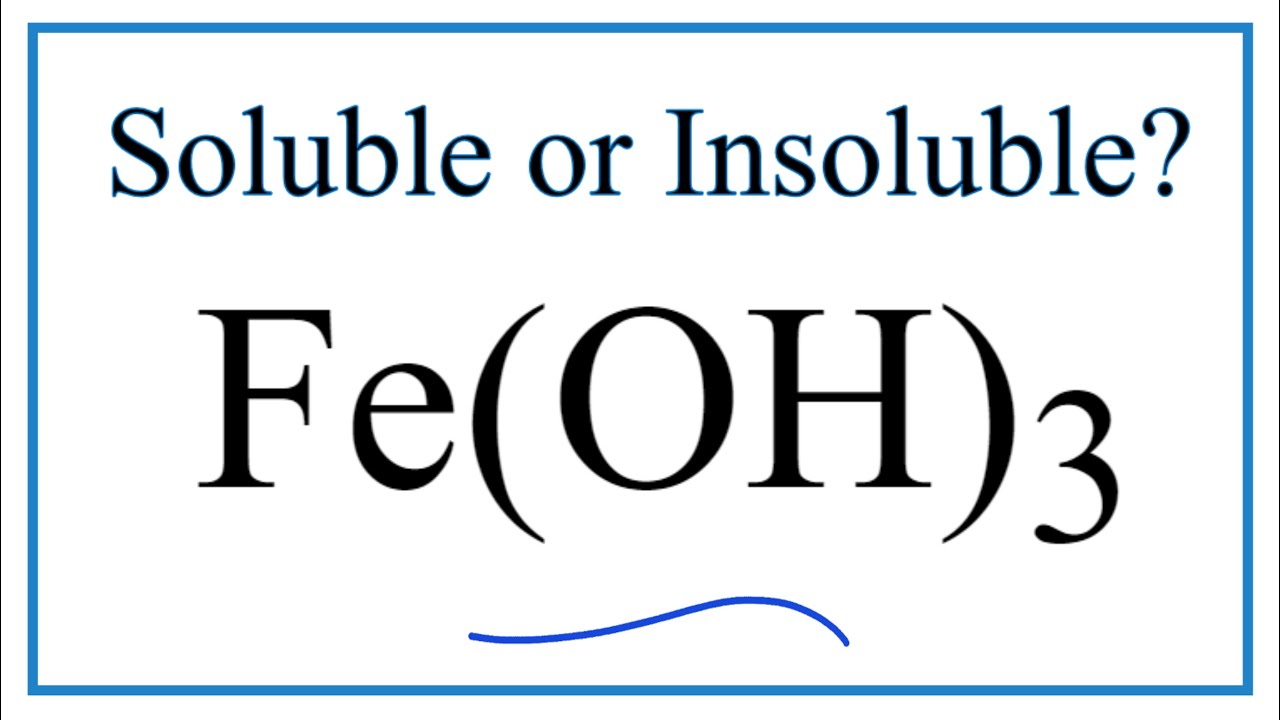
Is Fe Oh 3 Soluble Or Insoluble In Water Youtube

Iron Removal By Physical Chemical Way

How To Write The Net Ionic Equation For Fe Oh 3 H2so4 Fe2 So4 3 H2o Youtube

Is Fe Oh 2 Soluble Or Insoluble In Water Youtube

At What Ph Fe Ii And Fe Iii Will Precipitate
Solved 1 The Concentration Of Oh In A Saturated Solution Of Chegg Com
Equation For Fe Oh 3 H2o Iron Iii Hydroxide Water Youtube
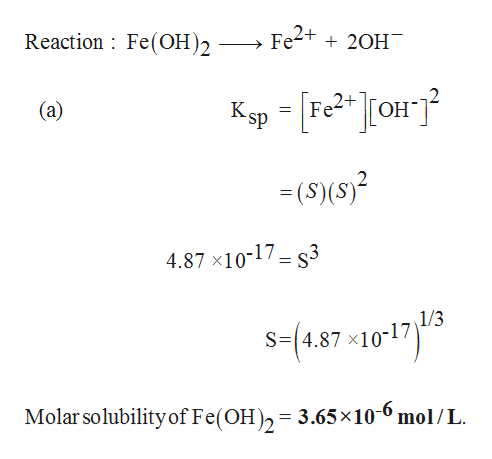
Answered The Ksp Of Fe Oh 2 Is 4 87 X 10 17 Bartleby
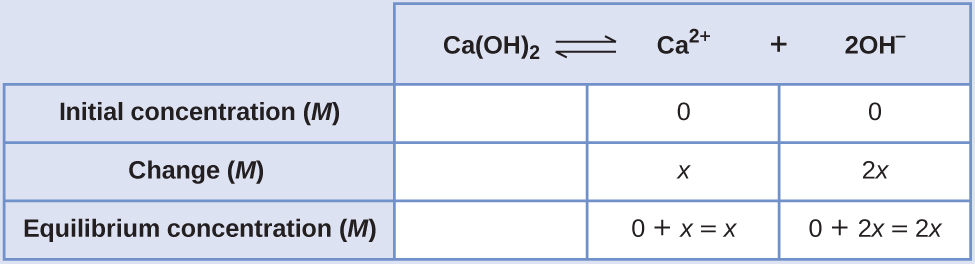
15 1 Precipitation And Dissolution Chemistry

How To Write The Equation For Fe Oh 2 H2o Iron Ii Hydroxide Water Youtube

What Is The Molar Solubility Of Fe Oh 3 In A Solution With A Hydroxide Ion Concentration Of 0 05 Quora

Inorganic Chemistry Is Fe Oh 2 Soluble In Alkalis Chemistry Stack Exchange

Calculate The Molar Solubility Of Ni Oh 2 When Buffered At Ph 8 0 Lisbdnet Com
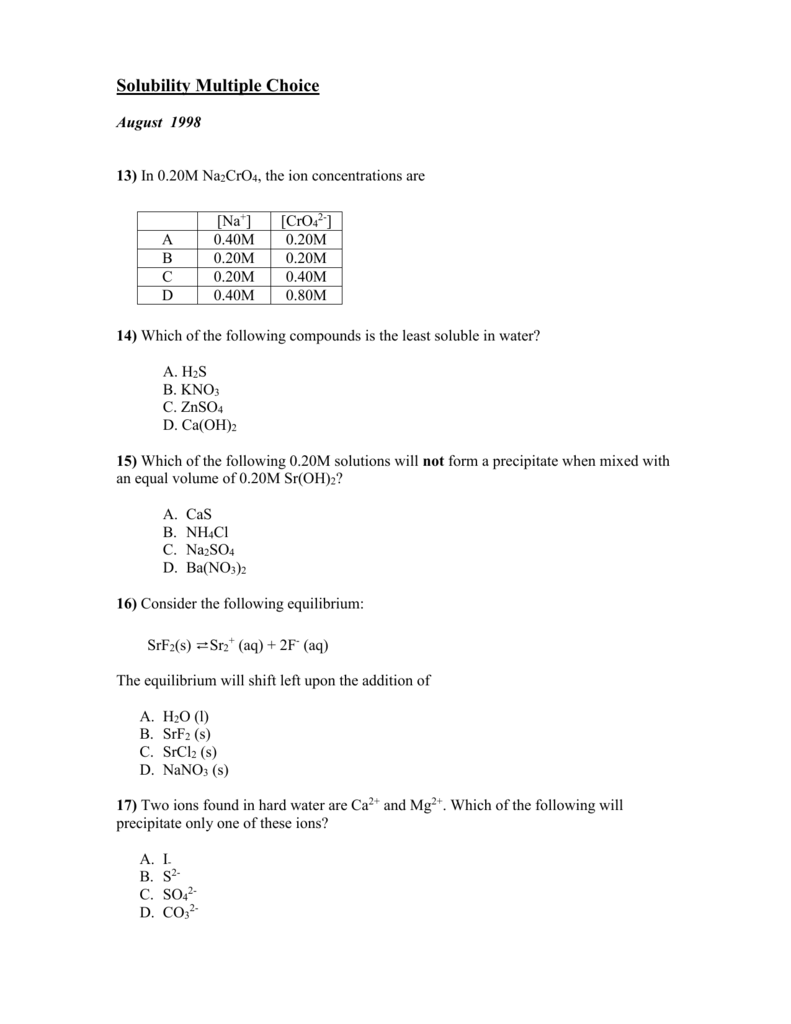

Comments
Post a Comment